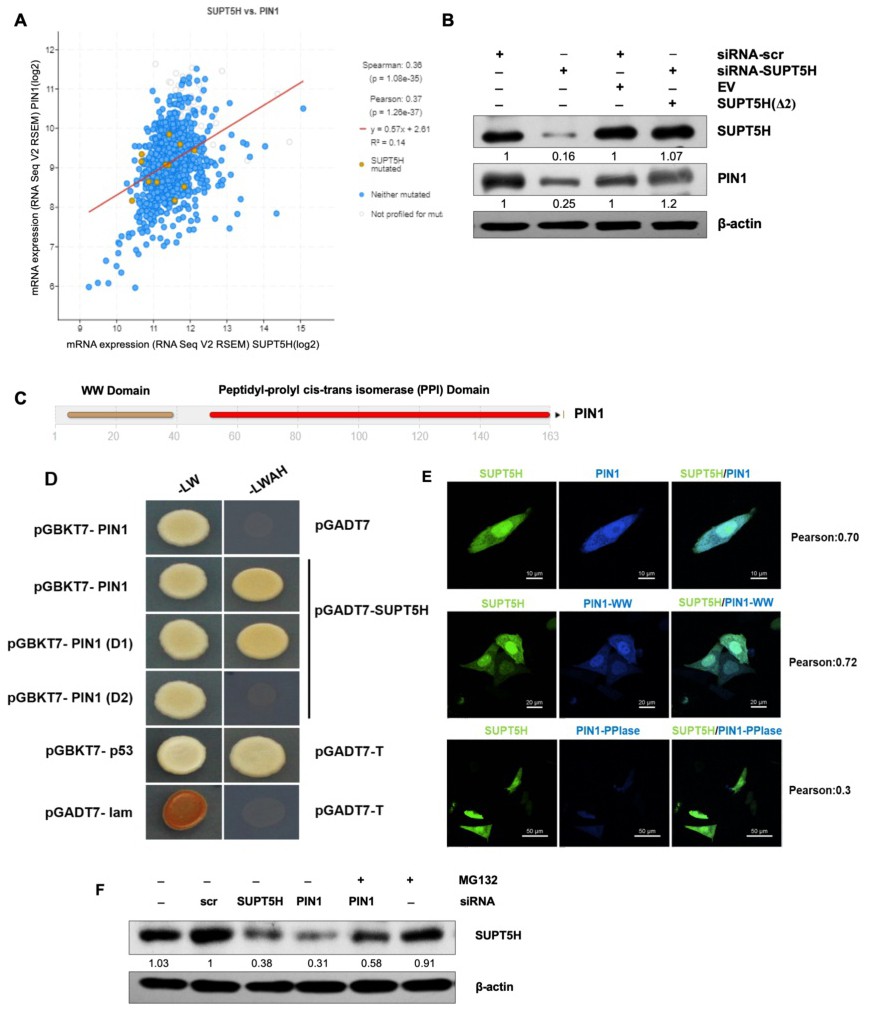
Fig. 2. Co-expression correlation and stability landscape of interaction between SUPT5H and PIN1. (A) Dataset from cBioportal showed a positive correlation between SUPT5H and PIN expression in breast cancer. (B) Western blot of SUPT5H and PIN1 proteins in MDA-MB-231 cells transfected with siRNA-SUPT5H only, siRNA-SUPT5H plus the siRNA-SUPT5H resistant ORF construct SUPT5H(Δ2), non-silencing siRNA-scr or siRNA-scr plus empty vector (EV) (representative blots are shown from two independent experiments). The quantitative comparison of siRNA-scr v/s siRNA-SUPT5H and siRNA-scr+EV v/s siRNA-SUPT5H+SUPT5H(Δ2) was performed. (C) A 163 residue protein PIN1 with two domains: WW domain and PPI domain (D) Interaction of pGBKT7-PIN1 with pGADT7-SUPT5H and pGBKT7-PIN1WW domain with pGADT7-SUPT5H were detected by DDO (-LW) and QDO (-LWAH) selection using yeast two-hybrid screening. Vectors pGBKT7-p53 with pGADT7-T and pGBKT7-Lam with pGADT7-T were used as a positive and negative control, respectively. The possibility of false-positive was eliminated by confirming that the activation domain in the empty vector could not interact with the pGBKT7-PIN1 bait vector. (E) MDA-MB-231 cells were co-transfected with mtorquois-PIN1 and mVenus-SUPT5H, mtorquois-PIN1WW and mVenus-SUPT5H, and mtorquois-PIN1PPIase and mVenus-SUPT5H plasmids. The colocalization of signals were visualized and analyzed using confocal microscopy. (F) MDA-MB-231 cells were transfected with siRNA-scr, siRNA-SUPT5H, or siRNA-PIN1. About 48h later, cells were further treated with MG132 or DMSO for 8h. Western blotting examination indicated the role of proteasome on PIN1 regulation of SUPT5H abundance (representative blots are shown from two independent experiments).